Chemical Structure:
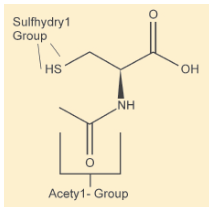
N-Acetyl Cystein is the acetylated precursor of both the amino acid L-cysteine and reduced glutathione N-acetylcystein is a sulfthydryl-cantaining compound. The biological activity of N-acetylcystein is attributed to its sulfhydryl group, while its acetyl substituted amino group affords its protection against oxidative and metabolic processes.
Benefits of N-Acetyl Cysteine in Liver:
• N-acetylcysteine stimulates glutathione synthesis, promotes liver detoxification by inhibiting xenobiotic biotransformation, and is a powerful nucleophile capable of scavenging free radicals.
Other Benefits of N-Acetyl Cysteine:
• N-acetylcysteine decreases expectoration difficulty, cough severity and diaphragm fatigue in respiratory illness.
• N-acetylcysteine is capable of enhancing T cell immunity.
• N-acetylcysteine reduces plasma homocysteine levels by 45%
 Clinical Evidences
Clinical Evidences
• N-Acetyl Cysteine modulates inducible nitric oxide synthesis gene expression in human hepatocytes.
Preliminary evidences that NAC might have hepatoprotective actions of potential relevance in chronic inflammatory liver diseases, medicated partially through the modulation of NO production.
J Hepitol. 2004 Apr;40(4):632-7
• NAC enhances the response to IFN in CHC.
J Interferon Res. 1993 Aug;13(4):279-82